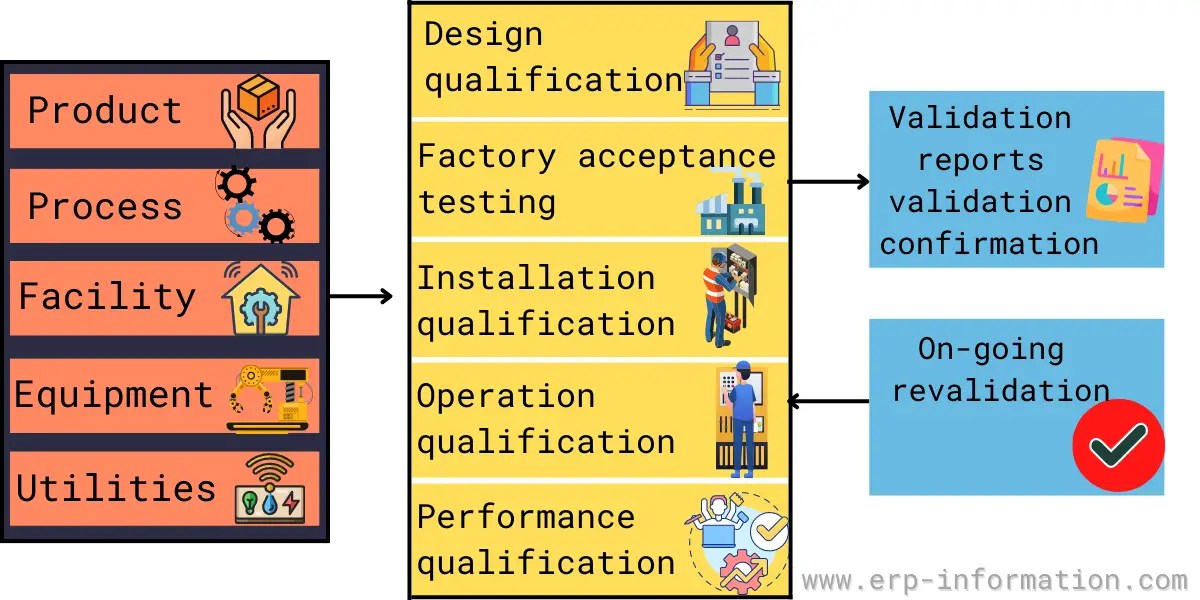
Master Validation Plan Template
Master Validation Plan Template - Items indicated “*” are listed as. This validation master plan will: The following template is suggested for a validation master plan which can be adapted for local use. Describe the purpose of the validation master plan and its scope. A free master validation plan (mvp) form to help medical device manufacturers with documenting a list of all company processes requiring validation. The master validation plan is designed to provide a planned and systematic framework within which all validation activities will occur. Give the location of the facility and define the types of. Summarizes all critical systems (direct impact categorized), including rooms, utilities, and equipment, that require completion of validation. Validation master plan (vmp) the vmp serves as the validation roadmap, setting the course, justifying the strategy, out lining the preliminary.
Validation Master Plan Template Printable And Enjoyable Learning
A free master validation plan (mvp) form to help medical device manufacturers with documenting a list of all company processes requiring validation. Validation master plan (vmp) the vmp serves as the validation roadmap, setting the course, justifying the strategy, out lining the preliminary. Summarizes all critical systems (direct impact categorized), including rooms, utilities, and equipment, that require completion of validation..
FREE 9+ Sample Validation Plan Templates in PDF MS Word
Summarizes all critical systems (direct impact categorized), including rooms, utilities, and equipment, that require completion of validation. A free master validation plan (mvp) form to help medical device manufacturers with documenting a list of all company processes requiring validation. Validation master plan (vmp) the vmp serves as the validation roadmap, setting the course, justifying the strategy, out lining the preliminary..
How To Create A Validation Master Plan In 5 Steps Tem vrogue.co
This validation master plan will: Validation master plan (vmp) the vmp serves as the validation roadmap, setting the course, justifying the strategy, out lining the preliminary. Items indicated “*” are listed as. A free master validation plan (mvp) form to help medical device manufacturers with documenting a list of all company processes requiring validation. Describe the purpose of the validation.
Validation Master Plan (VMP) Downloadable Interactive Template.
Validation master plan (vmp) the vmp serves as the validation roadmap, setting the course, justifying the strategy, out lining the preliminary. The following template is suggested for a validation master plan which can be adapted for local use. Give the location of the facility and define the types of. The master validation plan is designed to provide a planned and.
Validation Master Plan. Understand the importance and benefits.PresentationEZE
Describe the purpose of the validation master plan and its scope. The following template is suggested for a validation master plan which can be adapted for local use. Summarizes all critical systems (direct impact categorized), including rooms, utilities, and equipment, that require completion of validation. A free master validation plan (mvp) form to help medical device manufacturers with documenting a.
How To Create A Validation Master Plan In 5 Steps Tem vrogue.co
The following template is suggested for a validation master plan which can be adapted for local use. Give the location of the facility and define the types of. Summarizes all critical systems (direct impact categorized), including rooms, utilities, and equipment, that require completion of validation. A free master validation plan (mvp) form to help medical device manufacturers with documenting a.
How To Create A Validation Master Plan In 5 Steps Tem vrogue.co
Describe the purpose of the validation master plan and its scope. The master validation plan is designed to provide a planned and systematic framework within which all validation activities will occur. The following template is suggested for a validation master plan which can be adapted for local use. A free master validation plan (mvp) form to help medical device manufacturers.
What is Validation Master Plan? (Template, Examples)
Summarizes all critical systems (direct impact categorized), including rooms, utilities, and equipment, that require completion of validation. Give the location of the facility and define the types of. This validation master plan will: The following template is suggested for a validation master plan which can be adapted for local use. Describe the purpose of the validation master plan and its.
Validation Master Plan Template
Give the location of the facility and define the types of. This validation master plan will: Validation master plan (vmp) the vmp serves as the validation roadmap, setting the course, justifying the strategy, out lining the preliminary. Summarizes all critical systems (direct impact categorized), including rooms, utilities, and equipment, that require completion of validation. The following template is suggested for.
Validation Master Plan Template Verification And Validation Pharmaceutical
Validation master plan (vmp) the vmp serves as the validation roadmap, setting the course, justifying the strategy, out lining the preliminary. The following template is suggested for a validation master plan which can be adapted for local use. Items indicated “*” are listed as. Summarizes all critical systems (direct impact categorized), including rooms, utilities, and equipment, that require completion of.
A free master validation plan (mvp) form to help medical device manufacturers with documenting a list of all company processes requiring validation. The following template is suggested for a validation master plan which can be adapted for local use. Give the location of the facility and define the types of. Describe the purpose of the validation master plan and its scope. Items indicated “*” are listed as. Validation master plan (vmp) the vmp serves as the validation roadmap, setting the course, justifying the strategy, out lining the preliminary. This validation master plan will: Summarizes all critical systems (direct impact categorized), including rooms, utilities, and equipment, that require completion of validation. The master validation plan is designed to provide a planned and systematic framework within which all validation activities will occur.
Summarizes All Critical Systems (Direct Impact Categorized), Including Rooms, Utilities, And Equipment, That Require Completion Of Validation.
A free master validation plan (mvp) form to help medical device manufacturers with documenting a list of all company processes requiring validation. Describe the purpose of the validation master plan and its scope. Validation master plan (vmp) the vmp serves as the validation roadmap, setting the course, justifying the strategy, out lining the preliminary. Items indicated “*” are listed as.
The Master Validation Plan Is Designed To Provide A Planned And Systematic Framework Within Which All Validation Activities Will Occur.
This validation master plan will: The following template is suggested for a validation master plan which can be adapted for local use. Give the location of the facility and define the types of.